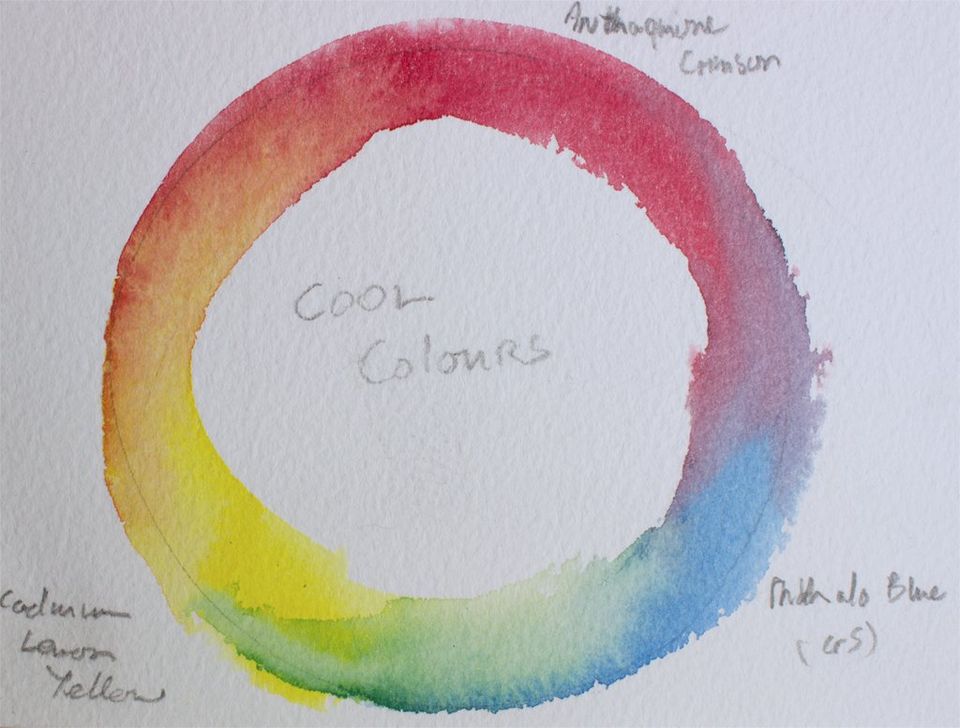ARTicles
Pigment Stories – Nickel Titanate Yellow, Cadmium Lemon Yellow and Cadmium Yellow
A periodic look at the stories behind the pigments used in A J Ludlow’s Professional Watercolours

Figure 1: Sketch of the Rutile crystal lattice, where the metal cations are found at each corner and one in the middle of the unit cell. Each metal cation is coordinated with 6 oxygen anions (in this sketch only the six oxygen atoms are shown coordinated to the central metal cation).
Incorporation of the Sb3+ and Ni2+ ions into the rutile (TiO2) lattice creates a pigment (NiO·Sb2O3·20TiO2) with a light lemon (green shade) yellow with high hiding power (the pigment’s opacity is a direct consequence of the rutile lattice) and so affords an opaque watercolour, which is semi-transparent in thin washes.
Figure 2: Graduated washes on white and black paper of Nickel Titanate Yellow Professional Watercolour and where the colour has been lifted out.
Although a muted colour (even to the point of being considered dull by some), Nickel Titanate Yellow is used to create dark flesh tones and by floral and flower artists (watercolour’s derived from this pigment are sometimes called “primrose yellow”), while some artists find it a very useful light yellow for inclusion in an “earth” palette.
Figure 3: Selection of colour mixes with Nickel Titanium Yellow and other watercolours in A J Ludlow’s Professional Range.
The pigment’s tinctorial strength is low, its staining power is also on the low side and the watercolour is easily lifted out. The watercolour’s lemon yellow hue produces attractive pastel mixes with violets and greens; with burnt sienna it produces a pastel brown, similar in shade to Naples yellow. Other names for this pigment include Sun Yellow, Titan Yellow and Nickel Rutile Yellow.
Although containing antimony and nickel, these metals’ bioavailabilty in the pigment used in our watercolour is very low and so the pigment is considered safe for use in our Professional watercolour range.
Cadmium Lemon Yellow:
Pigment Details: Cadmium Zinc Sulphide / Colour Index Pigment Yellow 35 (C.I. PY35)
Cadmium Lemon Yellow is prepared using a light lemon (green shade) mixed crystal inorganic pigment, cadmium zinc sulphide. The pigment is a solid solution of zinc sulphide in cadmium sulphide and its hue depends on the ratio of the two sulphides; the higher the amount of zinc sulphide the greener the cadmium yellow pigment will be. It therefore stands to reason that this pigment contains relatively more zinc sulphide than the cadmium zinc sulphide pigment used in my Cadmium Yellow watercolour.
Figure 4: Graduated washes on white and black paper of Cadmium Lemon Yellow Professional Watercolour and where the colour has been lifted out.
The pigment has excellent heat stability, a high degree of light fastness and chemical resistance, making it ideal for use in a professional watercolour range. On the artist’s palette, this colour is often used as a cool primary yellow.
Figure 5: Three colour cool primaries mixing circle, showing the colour mixes with Cadmium Lemon Yellow.
Although containing cadmium, this metal’s bioavailabilty in all the cadmium pigments we use in our watercolours is very low and so these pigments are considered safe for use in our Professional watercolour range.
Cadmium Yellow:
Pigment Details: Cadmium Zinc Sulphide / Colour Index Pigment Yellow 35 (C.I PY35)
Although the pigment cadmium sulphide (C.I. pigment yellow 37) has been synonymous with the colour name “cadmium yellow” since its introduction as an artists’ pigment in the early nineteenth century, the early forms darkened on exposure to air and light. Eventually a more stable yellow cadmium based pigment was found when cadmium sulphide was co-precipitated with zinc sulphide (C.I. pigment yellow 35) to produce a solid solution of zinc sulphide in cadmium sulphide.
My Cadmium Yellow Professional Watercolour is prepared using cadmium zinc sulphide, which as hinted above, contains less zinc sulphide in order to produce a redder cadmium zinc sulphide pigment. Cadmium based watercolours have a reputation for being on the opaque side, especially when applied in heavier layers, which can be used as an advantage when overpainting. In thin washes, my Cadmium based watercolours are bright and show good transparency due to the fact that only the pure pigment is used and the degree of pigmentation ensures a strong colour even when heavily diluted.
Figure 6: Graduated washes on white and black paper of Cadmium Yellow Professional Watercolour and where the colour has been lifted out.
The mixed crystal cadmium zinc sulphide pigment has excellent heat stability, a high degree of light fastness and chemical resistance. When used in watercolour, this pigment can be used on the artist’s palette as a brilliant mid-shade yellow, which can be mixed with blues to give a selection of bright greens or with reds to create warm oranges.
Figure 7: Three colour warm primaries mixing circle, showing the colour mixes with Cadmium Yellow.
***
I hope this ARTicle has been of interest to you. I will from time to time introduce the other pigments I use in my watercolours in future ARTicles, but in next month’s feature we will look at how to assess (and ultimately compare) watercolours and I will provide you with the test methods I use.