ARTicles
Pigment Stories – Phthalocyanine Blues and Greens
A periodic look at the stories behind the pigments used in A J Ludlow’s Professional Watercolours

Following on from my earlier ARTicles spotlighting the pigments used in my Professional Watercolour range, I would now like to turn our attention to the pigments derived from the organic complex, phthalocyanine, which I use in two blues, a turquoise and two greens.
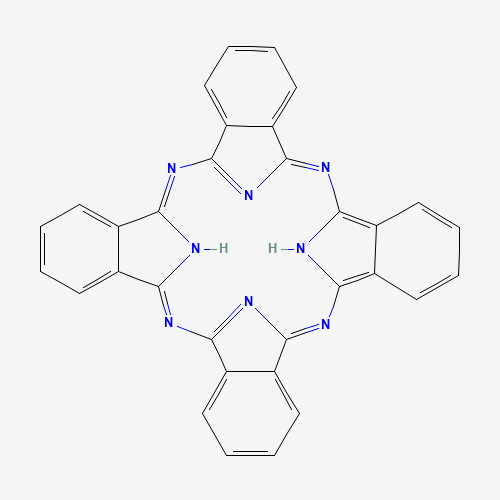
Figure 1: A 2D representation of the phthalocyanine macromolecule (PubChem)
The organic compound, phthalocyanine (pronounced thalo-si-a-neen) is a large, aromatic, macrocyclic, organic compound. It is composed of four isoindole units linked by a ring of nitrogen atoms (see figure 1). The extensive delocalization of the 18 conjugated π-electrons gives the molecule, amongst other properties, a blueish green colour which has been extensively exploited as an industrial colourant.
The phthalocyanine pigments form a large group of synthetic organic macromolecules that form macrocyclic ligand complexes and have a lot in common with the polyphyrins (a group of organic compounds that include the biochemicals chlorophyll and hemoglobin). The phthalocyanine anion can be complexed with various metals to form organic-metallic complexes. These complexes and the metal-free phthalocyanine molecule tend to have low solubility in common solvents and are thermally stable, with excellent light fastness. At least 70 different metal-phthalocyanine complexes have been synthesised, but the most common one is based on copper.
Although the first synthesis, which produced a blue powder bi-product are attributed to Braun and Tcherniac in 1907 and Diesbach and von der Weid in 1927, the pigment was not commercialised until 1935 when Imperial Chemical Industries announce their Monastral Fast Blue, followed by IG Farbenindustrie in 1936 and Du Pont in the late 1930s (Pigment Compendium, page 305), and the chlorinated derivative of phthalocyanine blue was first sold in 1938.
Phthalocyanine Turquoise
Pigment Details: Phthalocyanine (metal free) / Colour Index Pigment Blue 16 (C.I. PB16)
Unlike the phthalocyanine blue pigments (C.I. PB15:6 and C.I. PB15:4), the phthalocyanine turquoise is based on a metal free compound, which shifts the colour of the phthalocyanine chromophore from blue to a more greenish hue. The metal free phthalocyanine is not as robust as the organo-metallic phthalocyanines, having inferior heat stability and poorer chemical resistance, which limits its use in other applications, but its properties are fine for a watercolour pigment.

Figure 2: Graduated washes on white and black paper (showing the transparency) of Phthalocyanine Turquoise Professional Watercolour and where the colour has been lifted out.
This pigment, like the copper phthalocyanines are classed as organic pigments and as so, tend to have relatively smaller particle sizes and larger surface areas than inorganic pigments, giving them a tendency to have higher tinctorial strengths (the strength of the colour increases with decreasing particle size) and be more transparent (as the smaller particle size scatters less light), which can affect the cleanness of the resulting watercolour’s ability to “lift-out” and so watercolours based on organic pigments have a greater tendency to stain (as can be seen in figures 2, 3 and 4).
The consistency of Phthalocyanine Turquoise Professional Watercolour is highly structured, as are the other phthalocyanine pigmented watercolours in the range. This is due to their relatively larger surface area, which absorbs more liquid binder, thus affecting the watercolour’s rheology straight from the jar. Once diluted with water, the watercolours flow and paint smoothly and the high levels of pigmentation ensure that the colour is strong and intense.
Phthalocyanine Blue (Red and Green Shades)
Pigment Details (Red Shade): Epsilon (ε) - Copper Phthalocyanine / Colour Index Pigment Blue 15:6 (C.I. PB15:6)
Copper phthalocyanine exhibits polymorphism (existing in different crystal forms), the three commercially important ones are alpha (α), beta (β) and epsilon (ε); traditionally, red shade phthalocyanine blues were prepared from α-copper phthalocyanine (C.I. PB15:1 and C.I. PB15:2), whilst green shade ones from the β-form (C.I. PB15:3 and C.I. PB15:4). The ε-form is redder in shade, more stable and has a higher tinting strength than α-copper phthalocyanine, making this pigment the best choice for a red shade phthalocyanine blue in my professional watercolour range.

Figure 3: Graduated washes on white and black paper (showing the transparency) of Phthalocyanine Blues (red shade on the left, whilst the green shade is on the right) Professional Watercolour and where the colour has been lifted out.
Pigment Details (Green Shade): Beta (β) – Copper Phthalocyanine / Colour Index Pigment Blue 15:4 (C.I. PB15:4)
Commercially, the more stable β-copper phthalocyanine is easily converted from the α-form. Although both are formed of the planner copper-phthalocyanine complex, the crystal shape and structure is markedly different; the β-form is composed of a tightly packed herringbone structure, where the copper atom has octahedral geometry, resulting in rod shaped crystals, whilst the α-form is composed of a relatively more open crystal lattice, where the copper atom has square geometry and tends to form block crystals. This difference in crystal packing is the reason for the shift in colour and also why the α-form is less stable.
The greenish β-copper phthalocyanine is a very good clean transparent blue and can be used on the artist’s palette as a cool primary blue.
Phthalocyanine Green (Blue and Yellow Shades)
Pigment Details: Chlorinated Copper Phthalocyanine / Colour Index Pigment Green 7 (C.I. PG7)
Phthalocyanine Green (Blue Shade) Professional Watercolour is prepared using the synthetic halogenated aromatic macrocyclic organo-metallic pigment, copper polychloro-phthalocyanine, with 14 to 15 chloro-substituents at the 16 peripheral reactive sites on the molecule’s four isoindole units.
Pigment Green 7 is synthesised from copper phthalocyanine (the blue pigment), which can then be chlorinated in the presence of aluminium trichloride to form the green pigment, copper polychloro-phthalocyanine, which is usually a mixture of isomers and degrees of chlorination.
Copper polycloro-phthalocyanine has a bluish-green colour which is very similar in hue to Viridian (chromium (III) oxide dihydrate, C.I. pigment green 18) and is often used as a synthetic substitute, albeit in a fairly diluted mixture of pigment and other mineral fillers. In my Phthalocyanine Green (Blue Shade) Professional Watercolour, the pigment is pure and the tinctorial strength is very strong and so is best used sparingly when initially mixing with other colours.

Figure 4: Graduated washes on white and black paper of Phthalocyanine Greens (blue shade on the left, whilst the gyellow shade is on the right) Professional Watercolour and where the colour has been lifted out.
Pigment Details: Brominated Copper Phthalocyanine / Colour Index Pigment Green 36 (C.I. PG36)
The yellow shade green is achieved by partially substituting the chloro-substituents of copper polychloro-phthalocynine with bromine, forming copper polybromo, polychloro-phthalocyanine, with 4 to 9 bromo-substituents and 8 to 2 chloro-substituents at the 16 peripheral reactive sites on the molecule’s four benzene units (Pigment Compendium, page 305). Bromination is less efficient than chlorination and as a consequence the maximum substitution is 9 bromine atoms. The halogenated phthalocyanine (Pc) has the general formula of CuPc(Cl)x(Br)y, where the number of substituents (x+y) is approximately equal to 15.
Phthalocyanine Green (Yellow Shade) Professional Watercolour has a softer yellow shade of green than the Phthalocyanine Green (Blue Shade) and so can be mixed with various yellows and earth colours to produce natural looking greens for foliage and landscape painting. In particular with Isoindolinone Yellow it can be mixed to make a lightfast green with a similar hue to natural Sap Green, which traditionally is made from buckthorn berries.
References:
Eastaugh N, Chaplin T, Siddall R, Walsh V, “Pigment Compendium: A Dictionary and Optical Microscopy of Historic Pigments”, Routledge, Abingdon 2013
Pub Chem website accessed on 31/12/21
https://pubchem.ncbi.nlm.nih.gov/compound/phthalocyanine#section=Structures
****
I hope this ARTicle has been of interest to you. In next month’s feature we will shine the spotlight on another interesting aspect of watercolour painting.