ARTicles
Pigment Stories – Ultramarine and Cobalt Pinks, Manganese Mauve and Manganese, Cobalt and Dioxaine Violets
A periodic look at the stories behind the pigments used in A J Ludlow’s Professional Watercolours

Following on from my earlier ARTicles, spotlighting the pigments used in the yellow, orange and red watercolours in my Professional Watercolours range, I would now like to turn our attention to the pigments used in the two pinks, the mauve and three violets. However, before we do, I would just like to touch upon colour mixing and how it can affect the brightness of the colour, as a lot of watercolourists prefer to mix their own “violets"* using red and blue and why I believe this may compromise the watercolours' brightness.
*According to Wikipedia (“Purple”, accessed on 24/08/21), violet is a spectral colour with its own wavelength, whereas purple is a composite colour made by combining red and blue. The article also goes on to state that purples are regarded as a redder shade, whilst violets are bluer. So, strictly speaking, when mixing red and blue, the resulting colour is purple.
Colour Mixing and its affect on purity of colour
Pigments are coloured because they selectively absorb and transmit (rather than reflect) light at specific wavelengths in the visible spectrum.
Figure 1: Absorption and transmission of light in the visible spectrum to produce colour.
For example, when visible light is shone onto a yellow-coloured watercolour, only the wavelengths corresponding to the yellow part of the spectrum are transmitted, the rest are absorbed.
The pigment’s purity of hue is related to the shape of the pigment’s absorption spectrum; the sharper the absorption spectrum, the brighter and purer the colour.
Figure 2: Light absorption spectra of a bright yellow as compared to yellow ochre.
The intensity and purity of colour is governed by the sharpness of the light absorption spectra and the fewer additional absorption bands present in the visible range, the purer the hue. In this example, the sharp rise in absorption curve of the bright yellow is responsible for its brightness, in the case of yellow ochre, the absorption curve is overall not so steep and therefore the colour is distinctly duller. It therefore follows that in pigment mixtures each pigment will obviously absorb light and so there are a number of absorption bands, which broadens the spectra and so results in a distinctly duller hue.
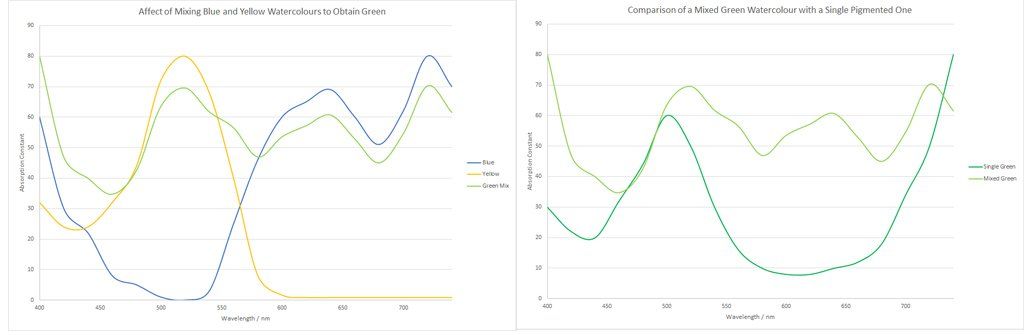
Figure 3 and 4: Spectra of a composite green and how it compares to a spectral green.
This is true for high-quality professional watercolours that are composed of mixed pigments. Mixing blue and yellow to make green, gives a fairly broad absorption spectrum, whilst a single pigmented green, has a well-defined absorption curve. Imagine the spectrum and brightness of a mixture of three pigments, or four, which unfortunately is not uncommon when the artist mixes watercolours together that are already composed of two pigments.
As in the example above, using a single pigmented violet will always have a purer hue and brightness than a similar hue produced by mixing red and blue.
Ultramarine Pink
Pigment Details: Sulphur containing Sodium Aluminosilicate / Colour Index Pigment Red 259 (C.I. PR259)
Ultramarine Pink Professional Watercolour is prepared using the synthetic mineral pigment based on the three-dimensional aluminosilicate lattice with a sodalite structure containing entrapped sodium ions and ionic sulphur groups (from “Pigment Compendium”, page 381).
Figure 5: Graduated washes on white and black paper of Ultramarine Pink Professional Watercolour and where the colour has been lifted out.
Chemically similar to the blue pigment (C.I. PB29), the colour of the red aluminosilicate-sodalite pigment (C.I. PR259), is due to the elemental constituents being in different ratios and hence their effect on the crystal lattice shape; for example, compositional analysis by Reckitt (Colours) Ltd gave the compositions (from “Artists’ Pigments”, page 57) as:
Blue Na6.88Al5.63Si6.35O24S2.4
Red Na5.88Al5.76Si6.21O24S2.52
The red pigment can be made from the blue, by treating with hydrogen chloride (dry hydrochloric acid gas) at high temperatures; sodium is stripped from the lattice as sodium chloride. Unlike the blue pigment, the C.I. pigment red 259 has poor tinting strength and so its colour is less intense. The pigment’s hue is quite unique and because of its relatively low colour strength offers the Artist a bright rosy pink, which can never be to dark. It can also be used in colour mixing, although it’s hue can easily be overpowered and so more subtle and delicate colour changes are available. Similar to Ultramarine Blue, Ultramarine Pink naturally granulates, creating interesting texture in the painting.
Cobalt Pink and Violet
Pigment Details: Triscobalt Bis(orthophosphate) / Colour Index Pigment Violet 14 (C.I. PV14)
Cobalt Pink and Cobalt Violet Professional Watercolours are prepared using two different hydrated forms of the inorganic metallic phosphate pigment, triscobalt (II) bis(orthophosphate), as the hue of cobalt phosphate violets vary with the degree of water of crystallisation.
The triscobalt bis(orthophosphate) octahydrate’s hue is pink, whilst the tetrahydrate is considered a deep violet (from “Pigment Compendium”, page 121). Both pigments are chemically stable and light fast.
The Cobalt Pink pigment available to the Victorian artist was based on cobalt magnesium oxide and, like Cobalt Red (also available at the time), was regarded as inferior and more expensive to the red pigments derived from the madder root. These cobalt pigments were considered chalky and have poor hues and so soon fell out of favour (from “Pigment Compendium”, page 121-122).
On the other hand, the hydrated cobalt phosphate has a soft pink hue, which is bright, unlike the “chalky” pink based on cobalt magnesium oxide. The resulting watercolour is transparent in thin washes and so enhances the pale pink hue with a luminosity derived from the whiteness of the watercolour paper. The pigment has a relatively high cost and low tinting strength, which has tended to reduce its popularity as an artists’ pigment.
Figure 6 and 7: Graduated washes on white and black paper of Cobalt Pink and Violet Professional Watercolours and where the colour has been lifted out.
Cobalt Violet is considered one of the traditional pigments for watercolour (Mayer 1991, page 136), even though its popularity as an artists’ pigment is somewhat affected by its relatively high cost and low tinting strength.
In order to counteract the low tinting strength of cobalt phosphate, A J Ludlow’s Cobalt Pink and Cobalt Violet Professional Watercolours have been formulated with a high level of pigment and because it is based on only pure pigment, the watercolour is bright and vivid in colour. Both watercolours granulate on painting out.
Manganese Mauve and Violet
Pigment Details: Ammonium Manganese (III) Pyrophosphate / Colour Index Pigment Violet 16 (C.I. PV16)
Manganese Mauve and Violet Professional Watercolours are prepared using the inorganic metallic phosphate pigment, ammonium manganese (III) pyrophosphate.
Figure 8 and 9: Graduated washes on white and black paper of Manganese Mauve and Violet Professional Watercolours and where the colour has been lifted out.
The shade difference is due to differences in the pigment’s crystal shape. The violet is derived from the blue shade α-polymorph, whilst the mauve is based on the mixed phase α and β-ammonium manganese (III) pyrophosphate. Attempts to synthesise the redder polymorph, β-ammonium manganese (III) pyrophosphate have always resulted in a solid solution of the 2 crystal phases, α and β. The redness of the manganese pigment used in Manganese Mauve Professional Watercolour is due to the low proportion of α -ammonium manganese (III) pyrophosphate present in the pigment’s crystal matrix.
Historically (from “Pigment Compendium”, page 257), “permanent mauve” was a colour term associated with manganese phosphate, which in Winsor & Newton’s 1892 catalogue, was listed as being made from “Phosphate of Manganese”, but other colourmen list the colour as being derived from other pigments; one describes “permanent mauve” as being a violet toned with “French Ultramarine”, whilst in 1898, Reeves stated that “permanent mauve” is synonymous with “permanent violet” and made with a “combination of alizarin”. Therefore because of this lack of consensus, it would appear that “permanent mauve” was not strictly synonymous with manganese mauve and vice versa.
The manganese violet colour name has been historically known as “permanent violet” and also “mineral violet”. The α-ammonium manganese phosphate (C.I. PV16) pigment is very similar in hue to that of the tetrahydrate form of cobalt phosphate (C.I. PV14), but has a higher tinctorial strength and so became more popular than the cobalt violet as an artists’ pigment.
The blueness of the manganese pigment used in Manganese Violet Professional Watercolour is due to the α -ammonium manganese (III) pyrophosphate present in the pigment’s crystal matrix, which is possible to synthesise without the red-shade β-polymorph.
Dioxaine Violet
Pigment Details: Carbazole Dioxazine / Colour Index Pigment Violet 23 (C.I. PV23)
Dioxaine Violet Professional Watercolour is prepared using the organic pigment, 8,18-Dichloro-5,15-diethyl-5,15-dihydrodiindolo(3,2-b:3',2'-m)triphenodioxazine (as given on the website of US National Library of Medicine). The pigment is a member of the dioxazine class of organic chemicals and as such has many of the inherent properties of an organic pigment.
Figure 10: Graduated washes on white and black paper of Dioxaine Violet Professional Watercolour and where the colour has been lifted out.
In general, organic pigments tend to have relatively smaller particle sizes and larger surface areas than inorganic pigments, giving them a tendency to have higher tinctorial strengths (the strength of the colour increases with decreasing particle size) and be more transparent (as the smaller particle size scatters less light), which can affect the cleanness of the resulting watercolour’s ability to “lift-out” and so watercolours based on organic pigments have a greater tendency to stain. The structured consistency of the highly pigmented Dioxaine Violet, like the other watercolours based on organic pigments in A J Ludlow’s Professional Watercolour range, is due the pigment’s relatively larger surface area, which absorbs more liquid binder, thus affecting the watercolour’s rheology.
The dioxaine pigment is a relative newcomer to the artists’ palette. Its origins can be traced back to 1928, when a patent was filed by researchers at the German Chemical Company, Hoeschst, detailing the synthesis of C.I. Pigment Violet 23, which up until the end of the Second World War, was only used to process diamine light blue, a direct dye for cotton (from the article published on 15 march 2009 on Paint & Coatings Industry website). It wasn’t until 1953 that the pigment, Hostaperm-Violet RL spec, started to be used in its own right and in combination with phthalocyanine blue pigments to produce lightfast reddish blue shades (from “The Origins of Colour”, page 20).
References:
Website: https://en.wikipedia.org/wiki/Purple accessed on 24 August 2021
Eastaugh N, Chaplin T, Siddall R, Walsh V, “Pigment Compendium: A Dictionary and Optical Microscopy of Historic Pigments”, Routledge, Abingdon 2013
Plesters J in “Artists’ Pigments, A Handbook of Their History and Characteristics”, Volume 2, Ashok R (Ed), Archetype Publications, London 1993
Mayer, R “The Artist’s Handbook of Materials and Techniques”, 5th Ed, Viking, New York, 1991.
Website of US National Library of Medicine accessed on 16/20/20: https://pubchem.ncbi.nlm.nih.gov/compound/C.I.-Pigment-Violet-23
Website of Paint & Coatings Industry (PCI) accessed on 22/10/20: https://www.pcimag.com/articles/88747-pigment-violet-23-marks-55-years-of-adding-color-to-the-world
Geißler G, “The Origins of Colour, The Development of Organic Pigments”, Hoechst AG Publication
***
I hope this ARTicle has been of interest to you. In next month’s feature we will shine the spotlight on another interesting aspect of watercolour painting.